Update on McGuff's 503B Outsourcing Facility for Compounded Drugs

MOS 503B Updates
January 2025
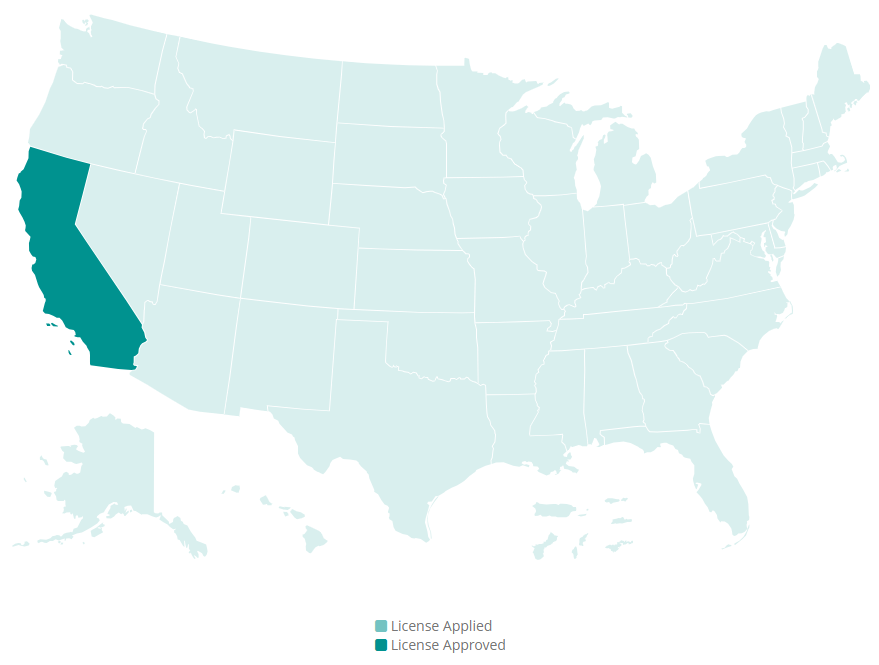
Since our last update, we’ve continued to work tirelessly and methodically to get our 503B Outsourcing Facility licensed with the California State Board of Pharmacy. Since it is such an involved and lengthy process, it takes time. Unfortunately, we’re unable to announce a precise launch date at this time, and we understand how frustrating that can be.
However, we want to reassure everyone that we remain steadfast to our goal and it’s our #1 priority. We are committed to providing healthcare providers with the compounded drugs they need to treat their patients.
The compounded drugs that we have produced that are currently in stability studies are:
- Alpha Lipoic Acid (ALA)
- Glutathione
- Methylcobalamin
- Procaine HCL
We do have exciting news about major renovations we've completed on our production facility's clean room while awaiting licensing. We have upgraded our HVAC units and ultra-low particulate air (ULPA) filtration systems, new in-line production testing equipment and have optimized production processes and scalability.
Our goal is to exceed cGMP standards, ensuring the highest quality compounded drugs on the market. McGuff stands apart from competitors through our commitment to proactive quality measures that maintain exceptional product consistency and reliability.
We will continue to post updates as we further progress in California licensing when more information becomes available.
Be sure to contact us with any questions or concerns: mos.answers@mcguff.com | (877) 444-1133.
August 2024:
Dear Valued Customers,
We hope this message finds you well. It has been a while since we have given you an update about our 503B outsourcing facility (OF) located in California. As many of you know, our journey to obtain California licensing has been a complex and rigorous process, and we are grateful for your continued support and patience.
Current Status
The good news is that our facility continues to be registered with the FDA and is allowed to manufacture the drugs you need under federal law. The uncertain aspect preventing us from launching sooner is obtaining our California Board of Pharmacy (CA BOP) 503B OF state license. We are still diligently working with the CA BOP to meet their sometimes unique regulatory expectations.
We are in a unique position compared to nonresident 503B OFs as they do not reside in California and may, upon licensing in their state, obtain licensing in all other states except California. Being a resident OF in California we can not apply for licensing in other states until we receive a California license. Being a California resident OF has become a distinct disadvantage when compared to other states.
Regardless of the obstacles we’ve faced, we’re committed to staying the course and optimistic for the future. We continue to remain at the forefront of the changing regulatory landscape and advocate for medical professionals. We believe wholeheartedly that what we’re attempting is hugely beneficial to the healthcare community and want to be the source of compounded drugs that doctors can trust and rely on for their patients!
A Journey of Dedication and Care
Building a 503B outsourcing facility is a significant endeavor that requires meticulous planning, rigorous inspections, and strict compliance with regulatory standards. Our team is dedicated to navigating these complexities to bring our vision to life. We understand that the uncertainty surrounding the timeframe can be challenging for you and your patients, and we appreciate your patience as we work through this.
Looking Ahead with Confidence
While we are not able to provide a set timeframe for when our licensing process will be completed, we are confident about the future. We are excited about the opportunities this new venture will bring, not only for our company but also for the healthcare community we serve.
Thank You for Your Patience
We cannot express enough how much we appreciate your patience and understanding during this time. Your trust and support have been invaluable to us, and we are eager to continue serving you with the highest standards of quality and service.
We will keep you updated as we make progress and as more information becomes available. In the meantime, please feel free to reach out to us with any questions or concerns. We are here to help and support you in any way we can.
Warm regards,
Ronald M. McGuff
President & CEO
McGuff Company Inc.
mos.answers@mcguff.com
![]()
McGuff Outsourcing Solutions 503B Outsourcing Facility Licensing Background
We’re in the process of upgrading to a 503B outsourcing facility and are looking forward to launching McGuff Outsourcing Solutions (MOS). MOS is the newest company in the McGuff Family of Companies. We are in the process of finalizing our facility inspection from the California State Board of Pharmacy (CA SBoP), which is a crucial step toward beginning operations and shipping compounded drugs to healthcare facilities and providers.
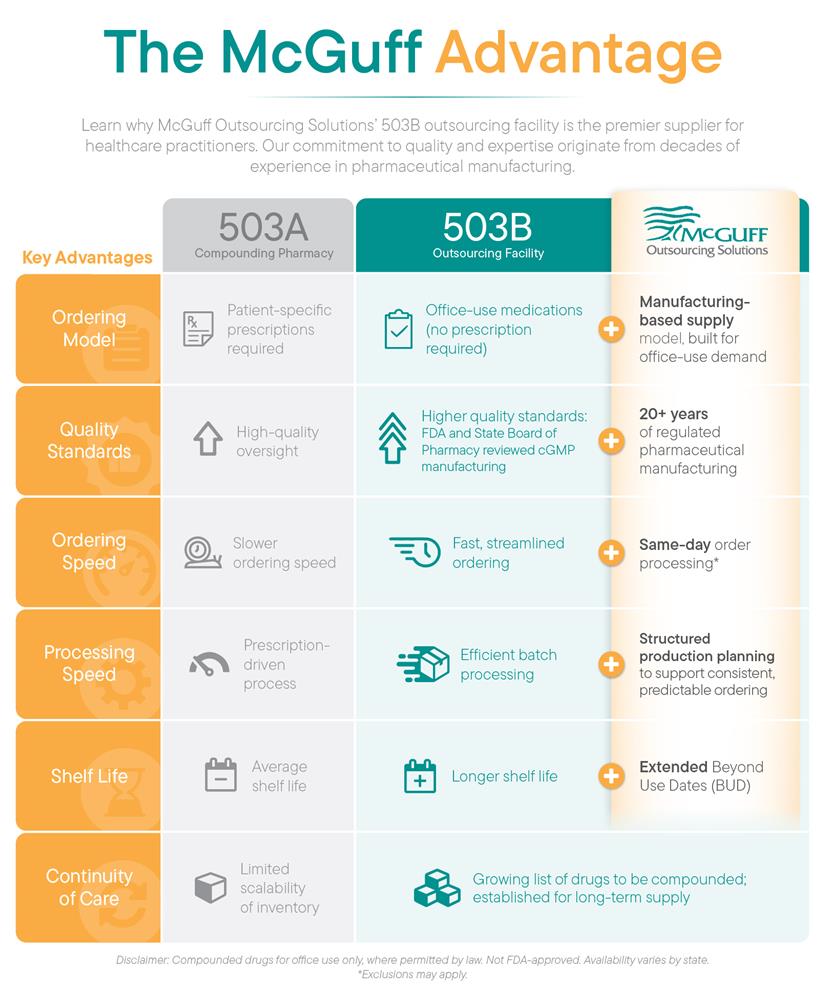
Previously operating as McGuff Compounding Pharmacy, a 503A, we look forward to continuing our 20+ years legacy of producing quality compounded drugs that medical providers can trust. The 503B designation, a mark of compliance and quality, allows us to produce large-scale compounded drugs under stringent FDA guidelines, meeting an urgent need for hospitals and clinics. The facility has already completed FDA registration and anticipates a quick turnaround on its CA SBoP application once inspected.
As an FDA-registered outsourcing facility, MOS can compound drugs for hospitals, clinics and office use. We can compound drugs from bulk drug substances that are on the FDA’s clinical need list, or that meet the criteria for inclusion on that list, or that appear on the FDA’s drug shortage list.
Benefits of 503B Outsourcing Facilities Compared to a Compounding Pharmacy:
- No prescriptions needed - order for office use
- Higher quality control and assurance
- Longer product shelf life
- Free, 3-day shipping
Upon license approval, we anticipate offering the following compounded drugs to start:
- Alpha Lipoic Acid (ALA)
- Glutathione
- Methylcobalamin
- Methione, Inositol, Choline Chloride (M.I.C.)
- Procaine HCL
We’ve been proactive in our efforts to prepare for the launch. Here are the key milestones we've achieved:
- FDA Registration Completion: Our facility has met the stringent requirements set by the FDA, ensuring we operate at the highest standards.
- Submission of CA State Board of Pharmacy License Application: This pivotal step was carefully prepared and submitted, bringing us closer to operational status.
- Response to CA State Board of Pharmacy's Additional Queries: We promptly addressed a few more questions from the CA SBoP, showing our commitment to transparency and compliance.
- Preparation for CA SBoP Inspection: We prepared and anticipated the CA SBoP's inspection, which validated our practices and procedures.
- Product Development Progress: Validation and stability batches of essential drugs like Glutathione and Methylcobalamin have been produced, with more in the pipeline.
Stay Informed of Our Progress
Thank You for Your Patience!
As we await final approval from the CA SBoP, we'd like to express our appreciation to our customers for their continued support. Be sure to bookmark this page to check back for ongoing updates, sign up to receive our emails and contact us if you have additional questions.
Register for Your Account
Also, if you haven’t already, register for your account at McGuff.com and you will automatically have an account set up with McGuff Outsourcing Solutions.
Are You a Patient?
If you are a previous McGuff Compounding Pharmacy patient who is looking for a pharmacy to fill your prescription, we will soon have a list of partner pharmacies for you to reference and will post the link here in this article.
Learn More About the Benefits of 503Bs